A Rapid, Secure Solution for Cardiac Device Management
A Rapid, Secure Solution for Cardiac Device Management
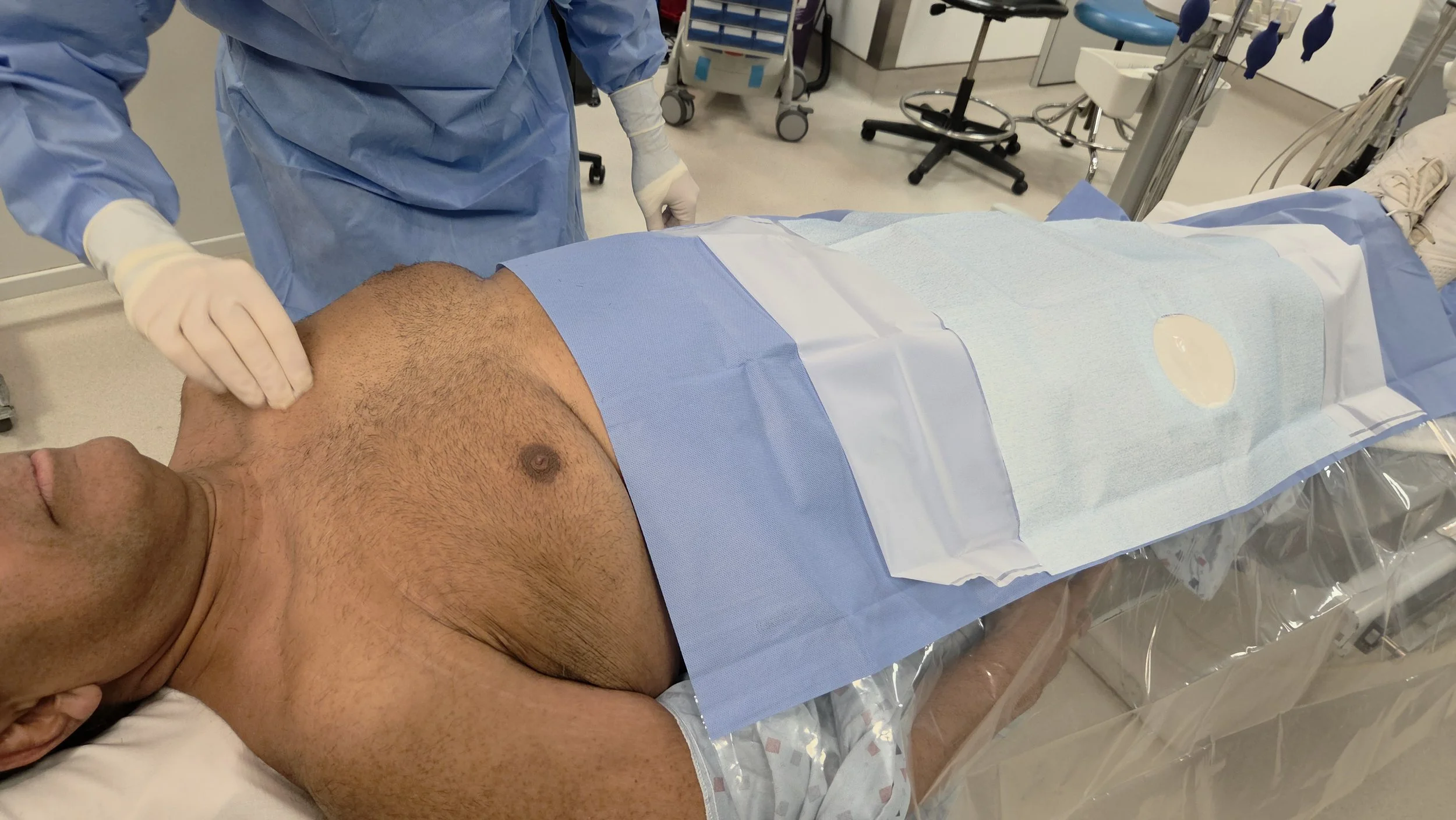
Cardiaguard™ is a single-use dermal patch designed to safeguard patients with implanted cardiac devices during surgical and diagnostic procedures.
Current Practices Leave Patients — and Hospitals — at Risk
Traditional EMI management methods rely on temporary device reprogramming or magnets that can delay procedures and increase risk. Cardiaguard eliminates these challenges with a simple, single-use solution.
Current Practice
Traditional approaches involve clinical magnets taped over devices, proving unstable and creating workflow challenges with inconsistent shielding.
With Cardiaguard™
Dedicated EMI-protective housing device providing consistent protection and seamless integration into surgical workflows.
| Traditional Approach | With Cardiaguard™ |
|---|---|
| Wait for rep to reprogram ICD/pacemaker before and after procedure | Immediate application by clinical staff – no rep needed |
| Potential EMI interference and pacing disruptions | Clinically validated EMI attenuation |
| Inconsistent shielding | Single-use medical-grade housing device |
| Workflow delays and coordination challenges | Seamless integration into surgical workflow |
| Limited documentation for risk mitigation | Supports compliance and procedural safety standards |
Cardiaguard provides consistent EMI protection — helping hospitals improve procedural efficiency, patient safety, and regulatory compliance with one simple step.
Simple & Effective
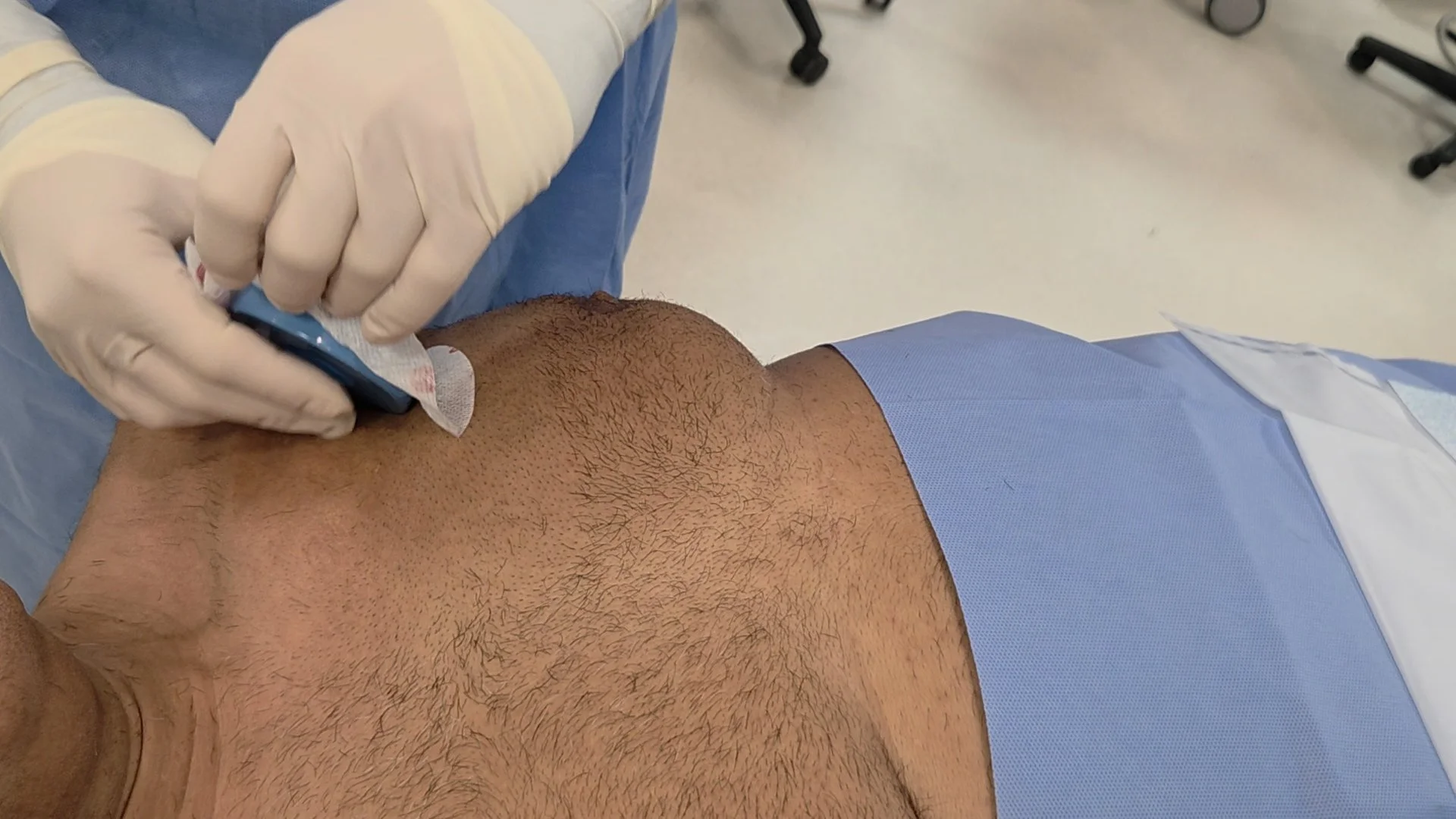
Clean the Application Area
Prepare the skin by wiping the implant site with a standard antiseptic solution to ensure proper adhesion.
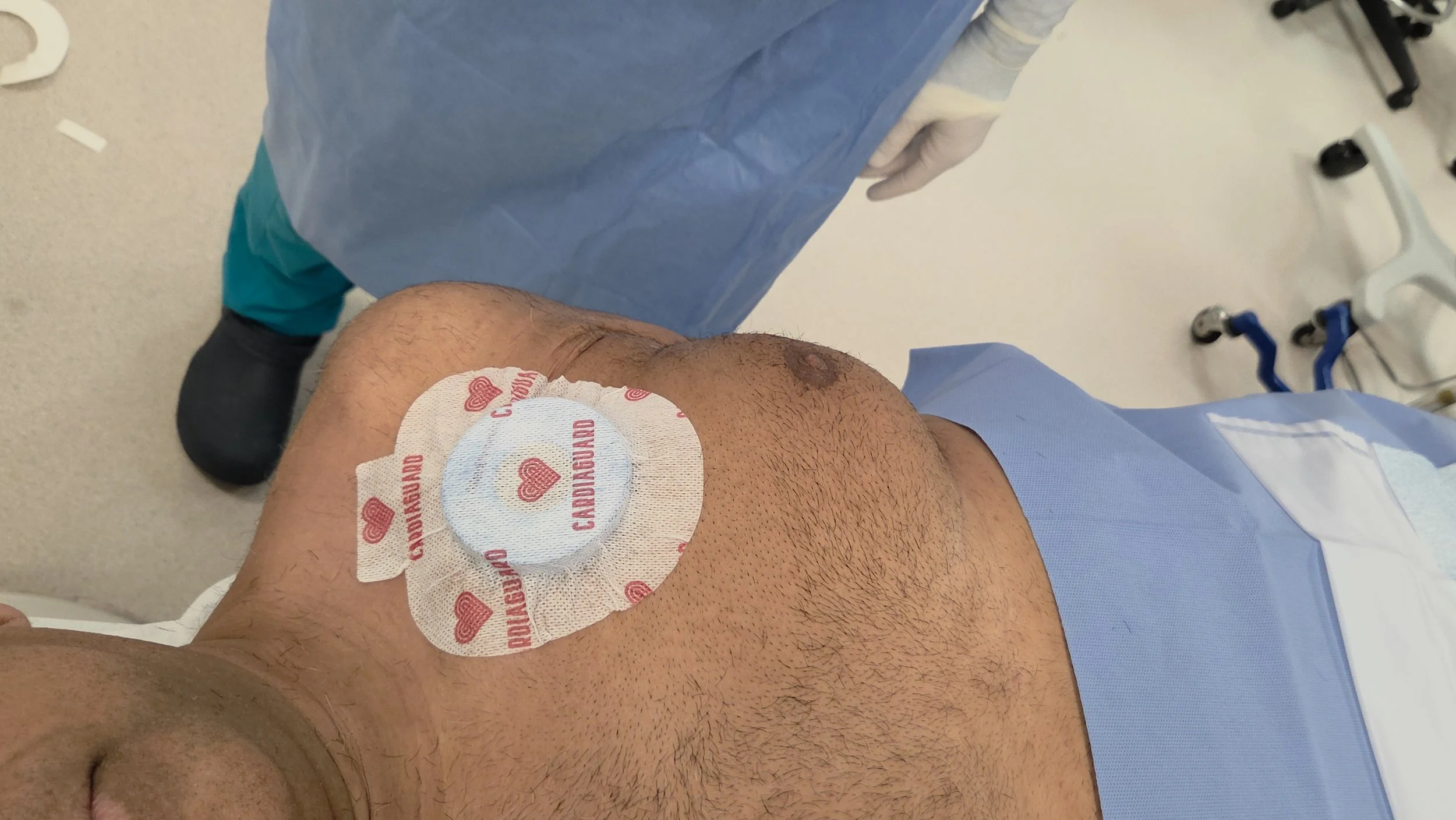
Apply Cardiaguard and Perform the Procedure
Place the Cardiaguard patch directly over the implant site. Once applied, proceed with surgery or imaging as normal with reduced EMI exposure.
Remove and Discard Post-Procedure
After the procedure is complete, gently remove the patch and discard according to standard clinical waste protocols.

Clinical & Operational Benefits
For Physicians
- Reduces EMI risk and device interference
- Maintains asynchronous pacing and ICD therapy inhibition
- Non-invasive, and disposable
- Integrates seamlessly into existing OR protocols
- Simplifies EMI management without workflow disruption
For Hospitals & Administrators
- Enhances compliance with peri-procedural safety standards
- Improves patient safety and procedural confidence
- Streamlines workflow – no setup, calibration, or equipment prep
- Reduces procedural complexity and downtime between cases
- Supports risk-reduction initiatives and staff training compliance
Backed by Data
Clinically Validated Performance
In pre-clinical and simulated procedural testing, Cardiaguard demonstrated reliable EMI protection with zero device interference events observed. Its proprietary multi-layer design delivers consistent shielding performance without compromising patient comfort or clinician workflow.
Backed by Data
Built for Clinical Confidence
Medical Device
Certified Manufacturer
Patent Approved
Quality Assured